Predictive value of the antibody titer
Anti-PLA2R autoantibodies can provide important information on the clinical activity of MN. Different studies have shown that disease course and therapy success in patients with pMN can be assessed by monitoring of the anti-PLA2R antibody titer.
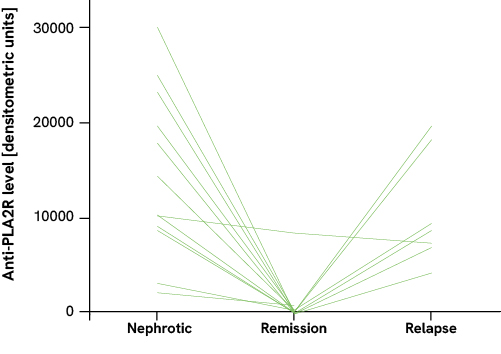
For example, patients with acute nephrotic syndrome (high proteinuria) showed high anti-PLA2R titers; with spontaneous remission or remission due to treatment (low proteinuria), however, the titers decreased to below the detection limit. In patients with initially high titers, spontaneous remission occurred much less frequently than in patients with low titers. Moreover, a relapse of the pMN was associated with an increase in the antibody level. From these observations, the authors concluded that the antibody level also reflects the severity of the pMN.1,2
The anti-PLA2R titer is also useful for therapy monitoring. In patients who successfully respond to immunosuppressive treatment, it decreases. In the event of a relapse of the disease, the antibody titer rises again.3,4 Moreover, a high anti-PLA2R titer was identified as a risk factor for patients not achieving a remission of proteinuria by the treatment.
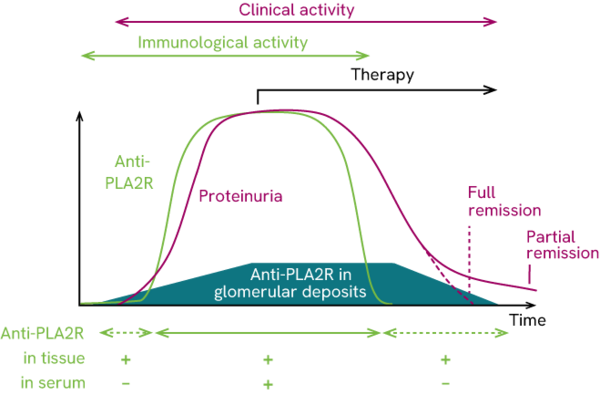
The study by Beck et al. further demonstrated that the immediate immunological response to therapy is significantly stronger than the clinical response. Thus, titer of anti-PLA2R autoantibodies in patients responding to immunosuppressive treatment decreased months before the reduction in proteinuria.3 Hoxha et al. came to the same conclusion: they found an 81% reduction in the anti-PLA2R titer of patient studied within 3 months after the start of immunosuppressive treatment. At the same time, the proteinuria decreased by around 39%.4
Despite the obvious link, the anti-PLA2R titer as an immunological marker should be observed apart from the proteinuria which acts as a clinical marker. Based on the chronological order of these events in the pMN pathogenesis known so far, it must be assumed that the markers react similarly over the disease course, but with a time lag.5
In around 40% of pMN patients, the disease reappears after kidney transplantation.6 The risk of recurrence is especially high if anti-PLA2R autoantibodies persist over a time period of six months after the organ transplant.7 As part of the risk assessment the titer can help to determine the necessity and intensity of immunosuppressive treatment after kidney transplantation in order to prevent a relapse.
CONCLUSION: It is recommended to always monitor the anti-PLA2R titer in parallel to the clinical indicator during the medical treatment of a pMN patient.8,9 Besides the relevance of the antibodies for the diagnosis of the kidney disease, the titer may provide further important information on the disease stage and the immediate therapy success. Moreover, some findings suggest that the anti-PLA2R titer can also be used as a predictive marker and allows assessment on the prognosis of MN, required therapeutic measures and the risk of another break-out of the disease after kidney transplant.
1Hofstra et al., Clin J Am Soc Nephrol 6, 1286-1291 (2011)
2Hofstra et al., Clin J Am Soc Nephrol 23, 1735-1743 (2012)
3Beck et al., J Am Soc Nephrol 22, 1543-1550 (2011)
4Hoxha et al., J Am Soc Nephrol 25, 1357-1366 (2014)
5Beck et Salant, Kidney Int 77, 765-770 (2010)
6El-Zoghby et al., Am J Transplant 9, 2800-2807 (2009)
7Seitz-Polski et al., Nephrol Dial Tranplant 0, 1-9 (2014)
8Hofstra et al., Nat Rev Nephrol 9, 443-458 (2013)
9KDIGO, Kidney inter. 100 (Suppl.): 1–276 (2021)