Recommendations for differential diagnosis
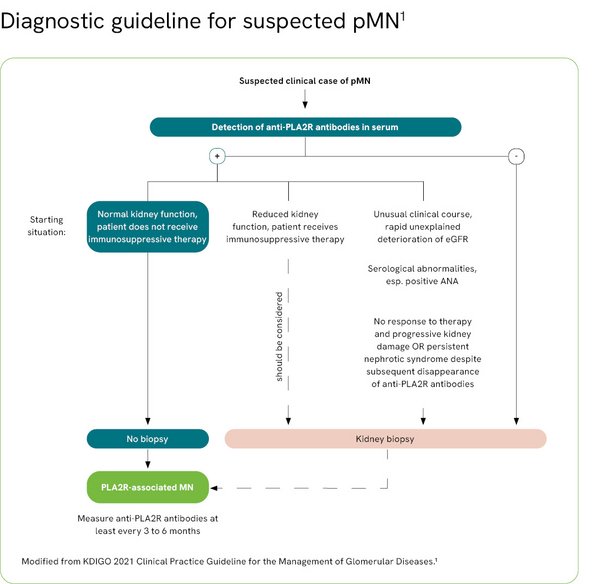
Depending on the course and severity of the disease, the new KDIGO global guideline now recommends a differentiated approach in pMN diagnostics. This is because the detection of anti-PLA2R antibodies confirm the diagnosis of pMN sufficiently clearly so that a biopsy can be avoided in most cases (see figure).1
The serological diagnostic approach is thus a non-invasive alternative to biopsy, requiring only a blood sample.
In addition to anti-PLA2R antibodies, anti-THSD7A antibodies represent a further marker in serological pMN diagnostics, which, due to its high specificity, like anti-PLA2R antibodies, is also ideally suited for differentiation from secondary MN. Moreover, simultaneous determination of anti-PLA2R and THSD7A antibodies increases the serological detection rate.2.3
Autoantibody detection by means of immunofluorescence, ELISA and chemiluminescence
EUROIMMUN has developed exclusive test systems based on recombinant antigens for precise determination of autoantibodies against PLA2R and THSD7A. The indirect immunofluorescence tests Anti-PLA2R IIFT (IgG) and Anti-THSD7A IIFT (IgG) use transfected cells expressing the antigens on their surface as standard substrates. For the enzyme-linked immunosorbent assay Anti-PLA2R ELISA (IgG), the recombinant receptor is biochemically purified from the transfected cells and then used to coat the microplates, while for the Anti-PLA2R ChLIA (IgG) magnetic particles are coated with the antigens. With these methods, the autoantibodies can be detected easily, quickly and highly specifically in a blood sample.
The immunofluorescence tests are reliable test systems that can be used for qualitative determination of autoantibodies against PLA2R and THSD7A. The Anti-PLA2R ELISA (IgG) and the Anti-PLA2R ChLIA (IgG) also allow quantitative determination of anti-PLA2R antibodies.
In addition to its usefulness in differential diagnostics of pMN, the autoantibody titer has a high predictive value with regard to
- Disease activity and course
- Therapy monitoring
- Prognostic prediction
- Risk assessment after transplantation
Options of automated processing
For all test systems, EUROIMMUN offers options for automation to match the laboratory workflow.
1. EUROIMMUN-ELISA automation solutions:
EUROLabWorkstation ELISA, Analyzer I, Analyzer I-2P and Sprinter XL
2. EUROIMMUN-IIFT automation solutions:
EUROLabWorkstation IFA, EUROPattern and EUROPattern Microscope Live
3. EUROIMMUN-ChLIA automation solutions:
IDS-i10, IDS-iSYS Multi-Discipline Automated System